Background: Despite the effectiveness of novel agents in treating CLL, prolonged single agent use can lead to drug resistance, toxicity, challenges with adherence, and considerable financial burden. Combinations may provide deeper prolonged remissions using defined treatment durations. Umbralisib (Umbra) is a novel and highly specific PI3Kδ/CK1ε dual inhibitor, while ublituximab (Ubli) is a glycoengineered monoclonal antibody targeting a unique epitope on CD20. A randomized Phase 3 study of the Umbra + Ubli (U2) combination in patients (pts) with treatment naïve and previously treated CLL, UNITY-CLL, was recently determined to have met its primary endpoint at a pre-planned interim analysis. Integrating the BCL2 inhibitor venetoclax (Ven) into the U2 backbone is hypothesized to reduce the risk of acquired drug resistance (Choudhary, Cell Death Dis 2015), achieve higher levels of undetectable minimal residual disease (MRD), and reduce the risk of tumor lysis syndrome (TLS). Phase 1 results established the recommended Phase 2 doses to be: Umbra 800 mg QD; Ubli 900 mg and Ven 400 mg QD (Barr et al., ASH 2019). The Phase 2 component evaluated the safety, efficacy and MRD negativity rates in relapsed or refractory CLL pts.
Methods: In Phase 2, pts received three cycles of Umbra 800 mg QD along with Ubli 900 mg, administered weekly during cycle 1 (Day 1/2 split 150/750), then on Day 1 in cycles 2 through 6. Ven is initiated in cycle 4 and increased in standard fashion to 400 mg and continues with umbralisib once-daily through cycle 12. The primary endpoint for Phase 2 was complete remission (CR) rate by iwCLL criteria. Undetectable MRD (<10-4 by 8-color flow cytometry) was a key secondary endpoint. Bone marrow (BM) and peripheral blood (PB) MRD undetectable pts stopped therapy after 12 cycles, while MRD detectable pts continued on single agent Umbra.
Results: 40 pts have been treated to date. Baseline demographics were as follows: male/female (26/14), median age 65 yrs (range 43-83), median # prior therapies 2 (range 1-5). 20 pts (50%) had prior ibrutinib of which 11 (55%) were BTK refractory with BTK resistance mutations found in 8 cases. High-risk genetic features included unmutated IGHV genes (n=20), del17p (n=8), del11q (n=11), TP53 mut (n=4), NOTCH1 mut (n=5) and SF3B1 mut (n=2). Baseline (screening) TLS risk amongst efficacy evaluable pts (n=34) was high (H), medium (M) and low (L) risk in 5, 19 and 10 pts respectively.
For all pts treated to date, the most common AEs were (all causality and all grade; >25% of pts) infusion related reactions [IRR] (63%), anemia (55%), thrombocytopenia (53%), neutropenia (53%), leukopenia (50%), creatinine increase, (50%), fatigue (45%), diarrhea (43%), nausea (38%), AST increase (30%). The only grade 3/4 AEs occurring in ≥ 5% of pts were neutropenia (23%) leukopenia (13%) and IRR (8%). Grade ≥ 3 PI3Kδ-associated toxicities of interest occurred in 2 pts (1 G3 colitis, and 1 G3 diarrhea). No TLS events were observed during Ven administration. Discontinuations of any study drug due to AE occurred in 3 pts, of which 1 pt discontinued all 3 agents (G3 diarrhea). Of 34 pts who completed 3 cycles of U2 debulking, TLS risk was substantially reduced: of 5 H-risk pts only 1 remained H-risk; of 19 M-risk pts, only 4 remained M-risk with the remainder at L-risk (79% reduction in H- and M-risk TLS).
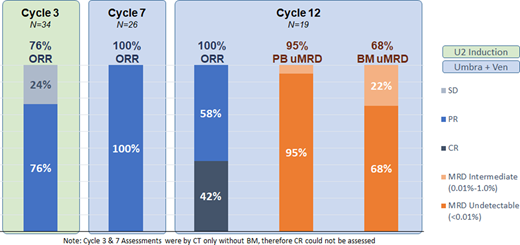
Among evaluable pts, the overall response rate was 76% (26/34) after cycle 3 (U2 debulking only), 100% (26/26) after cycle 7 and 100% (19/19) after cycle 12. Among the 19 pts who finished 12 cycles of therapy, 8 (42%) achieved CR by iwCLL criteria. Undetectable MRD (<0.01%) in the PB and BM was observed in 95% (18/19) and 68% (13/19) of pts respectively. MRD was intermediate (0.01% - <1.0%) in PB or BM in 6 pts past cycle 12 (Figure 1 ORR and MRD). Undetectable MRD (uMRD) in PB was maintained in 7/9 (77%) pts at cycle 18 and 4/5 (80%) pts at cycle 24. With a median f/u of 10.4 months (range 0.9-22.1), only 1 pt has experienced disease progression, 10 months after achieving PB and BM uMRD and stopping therapy.
Conclusion: U2-Ven is safe and highly effective in patients with relapsed/refractory CLL. Results suggest that this chemotherapy-free regimen can provide undetectable MRD after only 12 cycles of treatment, representing an effective 12-month treatment option for this population. These data have given rise to the multicenter ULTRA-V study, which is studying the U2-Ven triplet in patients with treatment naïve and relapsed/refractory CLL.
Barr:Seattle Genetics: Consultancy; Abbvie/Pharmacyclics: Consultancy, Research Funding; TG therapeutics: Consultancy, Research Funding; Verastem: Consultancy; Celgene: Consultancy; Merck: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Morphosys: Consultancy; AstraZeneca: Consultancy, Research Funding; Gilead: Consultancy. Ma:Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Kite: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Bioverativ: Consultancy, Honoraria; BeiGene: Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Research Funding; TG Therapeutics: Research Funding; Juno: Research Funding; Novartis: Research Funding. Zent:TG Therapeutics, Inc: Research Funding; Acerta / Astra Zeneca: Research Funding; Mentrik Biotech: Research Funding. Liesveld:Abbvie: Honoraria; Onconova: Other: data safety monitoring board. Sportelli:TG Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Miskin:TG Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Weiss:TG Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees. Friedberg:Acerta Pharma - A member of the AstraZeneca Group, Bayer HealthCare Pharmaceuticals.: Other; Roche: Other: Travel expenses; Seattle Genetics: Research Funding; Astellas: Consultancy; Bayer: Consultancy; Kite Pharmaceuticals: Research Funding; Portola Pharmaceuticals: Consultancy. Hill:Pharmacyclics: Consultancy, Honoraria, Research Funding; Beigene: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; AstraZenica: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Genentech: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Takeda: Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal